By America News World desk
Published: October 14, 2025 | Washington, D.C.

In a stark reminder of the global vulnerabilities in pharmaceutical supply chains, the World Health Organization (WHO) has issued a warning against three contaminated cough syrups manufactured in India. This alert comes weeks after the tragic deaths of at least 22 children in Madhya Pradesh, India, where lab tests revealed dangerously high levels of toxic chemicals in one of the implicated products. The incident has sparked international concern, prompting health authorities worldwide to scrutinize their imports, though U.S. officials have confirmed no shipments of these syrups reached American shores.
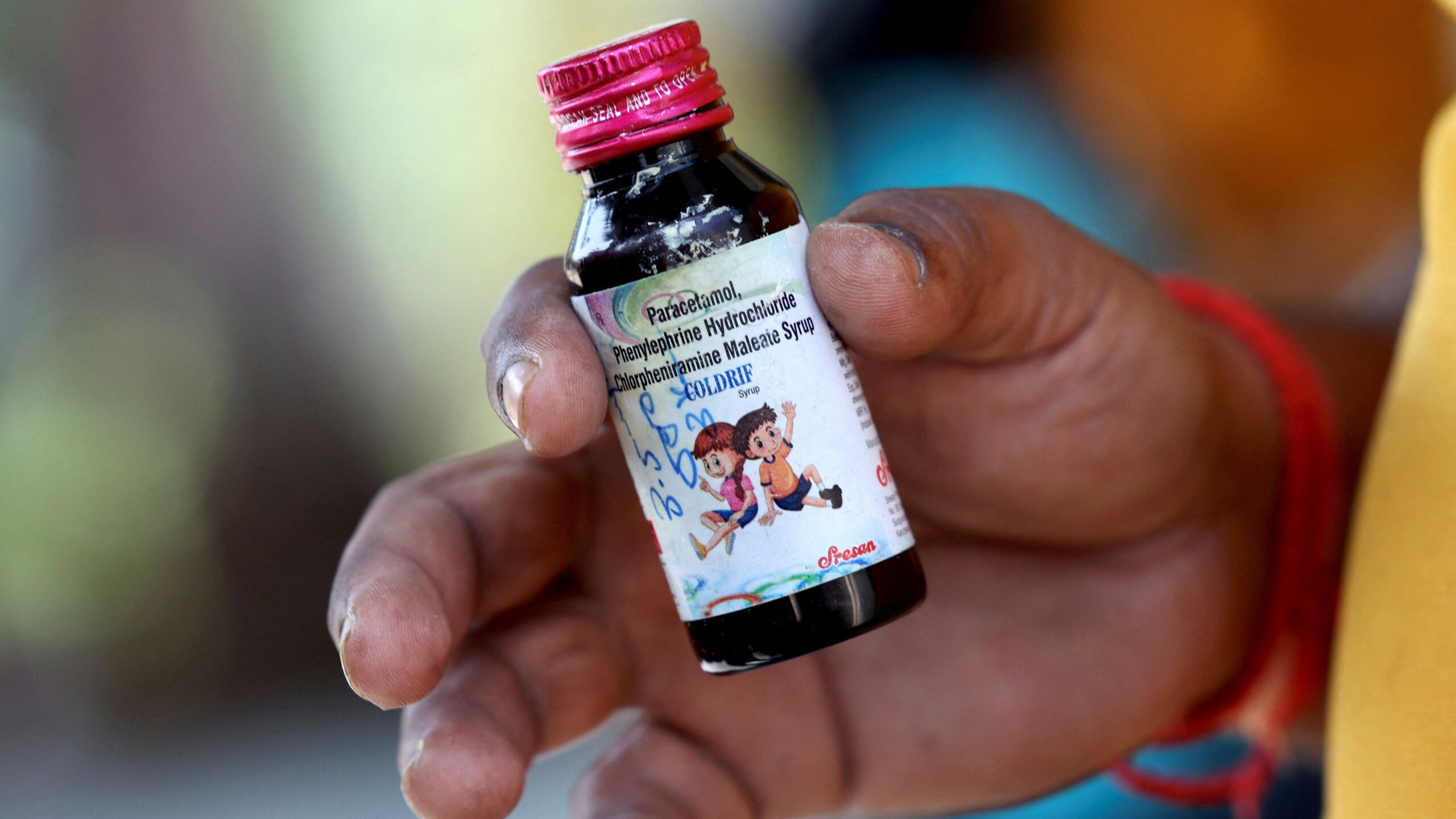
The WHO’s advisory, reported by Reuters, highlights specific batches of Coldrif from Sresan Pharmaceuticals, Respifresh TR from Rednex Pharmaceuticals, and ReLife from Shape Pharma as posing “significant risks” that could lead to severe illness or death. These syrups were found to contain diethylene glycol (DEG), a toxic industrial solvent sometimes used as a cheaper alternative to safer ingredients like glycerin in pharmaceuticals. DEG has a notorious history, linked to mass poisoning events across the globe, including fatal outbreaks in Panama, Nigeria, and Gambia in recent years.
The epicenter of this crisis is Parasia village in Chhindwara district, Madhya Pradesh, where young children—mostly under five years old—began falling ill after being prescribed cough syrup for common ailments like colds and respiratory issues. Symptoms escalated rapidly: abdominal pain, vomiting, acute kidney injury, and in the worst cases, coma and death. Local health officials initially attributed the deaths to a mysterious illness, but forensic analysis pinpointed Coldrif syrup as the culprit. Tests conducted by India’s Central Drugs Standard Control Organization (CDSCO) revealed DEG concentrations exceeding 48%—nearly 500 times the permissible limit of 0.1%.
Sresan Pharmaceuticals, based in Tamil Nadu, has borne the brunt of the backlash. The company’s manufacturing license was fully revoked following the uproar, and its owner, G. Ranganathan, was arrested on charges related to negligence and potential manslaughter. Raids on the firm’s facilities uncovered substandard production practices, including inadequate quality controls and possible substitution of ingredients to cut costs. In response, Tamil Nadu authorities have launched inspections of other drug manufacturers in the state to prevent similar lapses.
The WHO’s involvement escalated when Indian authorities reported the findings. The global health body inquired whether any of the contaminated batches had been exported, fearing a repeat of past international scandals. According to PTI, India assured the WHO that no exports occurred, a claim corroborated by U.S. health officials who stated that none of the toxic syrups entered the American market. This reassurance is crucial for the U.S., where imported pharmaceuticals undergo rigorous FDA scrutiny, but it underscores the broader risks in global drug trade, especially from regions with varying regulatory standards.
Diethylene glycol poisoning is not new to India. Historical incidents, such as the 1986 Mumbai tragedy where over 14 people died from contaminated glycerin, highlight recurring issues in the country’s vast pharmaceutical industry—valued at over $40 billion and a major exporter of generic drugs. Critics argue that lax enforcement, counterfeit drugs, and profit-driven shortcuts exacerbate these problems. The Madhya Pradesh case has reignited calls for stricter regulations, including mandatory testing for toxic impurities and better traceability in supply chains.
In the aftermath, the Indian government issued a nationwide advisory cautioning against prescribing cough syrups to children under two and recommending against their use for those under five unless absolutely necessary. States and union territories have been urged to enhance vigilance, with drug inspectors conducting spot checks on pharmacies and manufacturers. Public health campaigns are also underway to educate parents on safer alternatives, such as saline nasal drops or honey-based remedies for older children.
From a U.S. perspective, this incident serves as a cautionary tale amid ongoing debates about drug safety and importation. The FDA has long warned about the dangers of unregulated imports, particularly from online pharmacies or unverified sources. While the U.S. market remains unaffected, American parents and healthcare providers are reminded to verify product origins and consult pediatricians for over-the-counter remedies. Organizations like the American Academy of Pediatrics emphasize evidence-based treatments, steering clear of syrups with potential risks for young children.
The WHO’s alert is part of a broader effort to combat substandard and falsified medical products, which claim hundreds of thousands of lives annually, mostly in low- and middle-income countries. The agency has called on member states to report any detections of these syrups and to strengthen pharmacovigilance systems. Collaborative initiatives, such as the WHO’s Global Surveillance and Monitoring System, aim to detect and respond to such threats swiftly.
As investigations continue, families in Madhya Pradesh grieve their losses while demanding justice and compensation. Community leaders in Parasia have organized vigils and petitions, pressing for accountability from both manufacturers and regulators. The tragedy has also spotlighted socioeconomic factors: many victims came from impoverished rural areas with limited access to quality healthcare, relying on affordable but risky local medicines.
read this-Haryana DGP Shatrujeet Kapur sent on leave amid IPS officer’s suicide row
This episode echoes the need for international cooperation in pharmaceutical oversight. As globalization intertwines supply chains, ensuring drug safety requires unified standards and rapid information sharing. For now, the WHO’s warning stands as a critical safeguard, potentially averting further tragedies worldwide.
In the U.S., where cough syrups are a staple in many households, experts advise sticking to FDA-approved brands and monitoring for recalls via the agency’s website. Parents should watch for signs of poisoning—such as unusual lethargy or urinary issues—and seek immediate medical help if suspected.
As the world watches, India’s response could set precedents for handling similar crises. With ongoing lab analyses and legal proceedings, more details may emerge, but the human cost—22 young lives lost—remains a poignant call for reform
Stay updated with America News World for the latest on global health alerts, U.S. impacts, and international news. Subscribe to our newsletter for real-time updates on stories that matter.
Discover more from AMERICA NEWS WORLD
Subscribe to get the latest posts sent to your email.































Leave a Reply